Introduction
- Cardiogenic Shock (CS) is a type of shock identified by inadequate cardiac output to meet the tissue oxygen delivery requirements.
- CS is associated with high rates of morbidity and mortality
- CS is very challenging to identify and treat because presentation and treatment is different from other forms of shock (i.e. septic shock).
- AMI –> accounts for 81% of cases
Definition of Cardiogenic Shock
Causes
- “CS is a low-cardiac-output (CO) state resulting in life-threatening end-organ hypoperfusion and hypoxia”
- Overall, “leads to a state in which ineffective CO caused by primary cardiac disorder results in both clinical and biochemical manifestations of inadequate tissue perfusion”
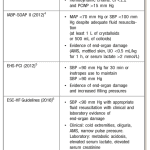
- No standard diagnostic criteria, but various trials used variations of criteria (see image on the right).
Any condition that decreases cardiac output can cause CS:
- Myocardial
- **Ischemic heart disease** (i.e. Acute myocardial infarction, or chronic deteriorating heart failure)
- Acute inflammation (sarcoidosis, myocarditis)
- Valves – Acute/chronic valve dysfunction
- Pericardium – Pericardial tamponade/constriction
- NOTE: PE must be ruled out
Hemodynamics
When the patient develops acute CS, cardiac output drops, resulting in reduced tissue perfusion. This activates compensatory mechanisms resulting in:
- Hypotension
- Vasoconstriction (increased catecholamines)
- Salt/water retention (renal/adrenergic activation of Renin-Angiotensin System).
- Sharp rise in preload
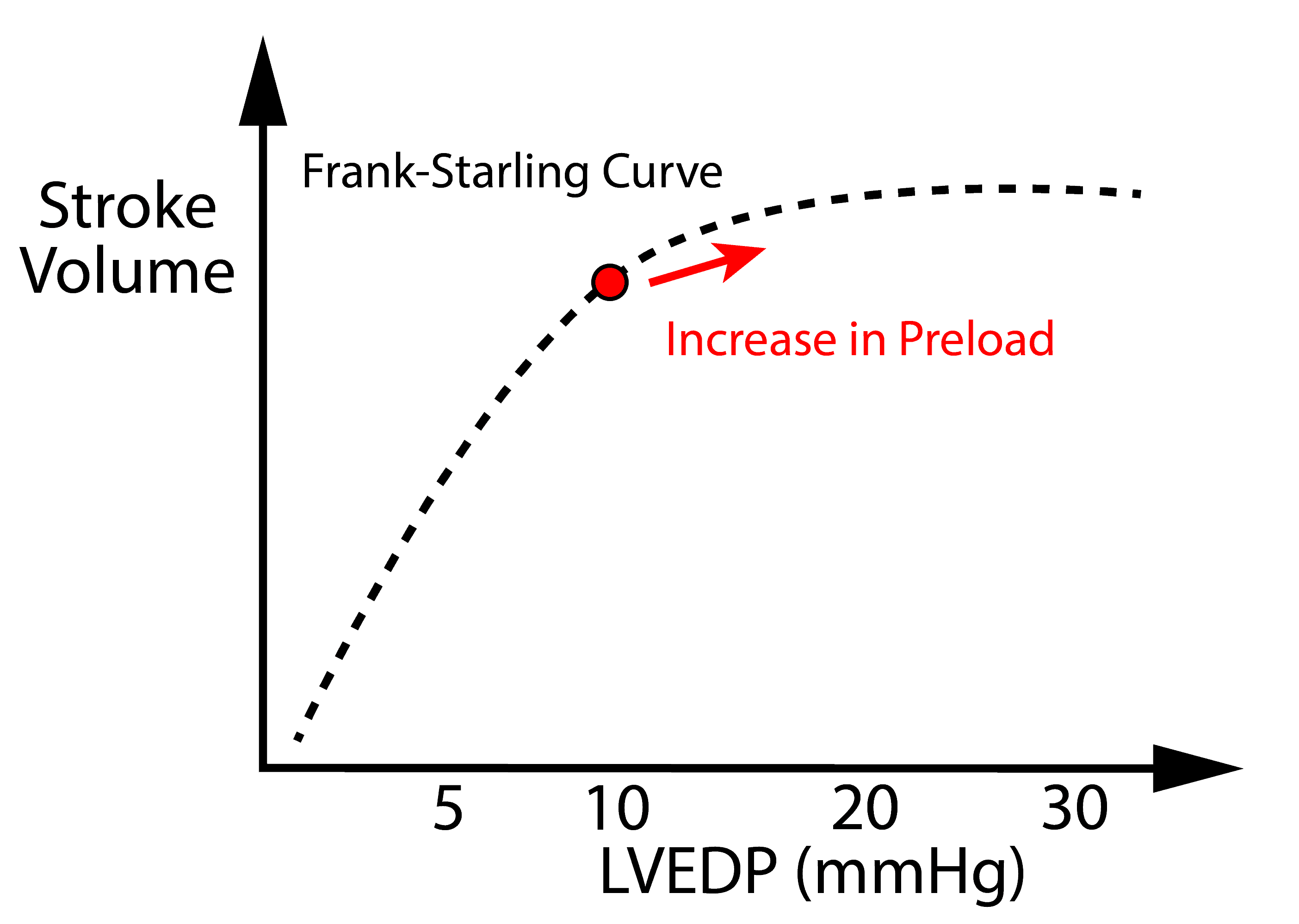
Preload is initially beneficial because it increases cardiac output to a certain extent. An increase in preload has diminishing returns – see Frank Starling Curve.
However, the downside of preload is venous congestion:
- Left-sided venous congestion –> Pulmonary edema –> Hypoxemia
- Right-sided venous congestion –> End-organ high venous pressure –> reduction of end organ perfusion due to a drop in tissue perfusion pressure (MAP – CVP)
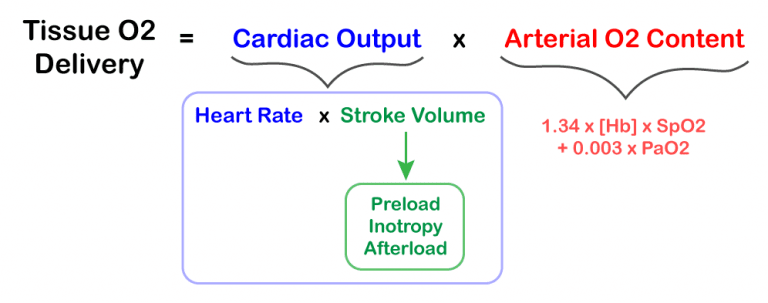
- Tissue oxygen delivery is not directly related to blood pressure (see formula on the right).
- Blood pressure is inversely correlated to systemic vascular resistance (SVR)
- In patients with CS, cardiac output is generally fixed. Tissue perfusion is reduced, leading to compensatory vasoconstriction. This increases systemic vascular resistance (SVR), resulting in increased BP.
- NOTE: Although BP is reduced in most patients with CS, a subset of patients with CS experience significant vasoconstriction (rise in SVR), which increases blood pressure to a normal level. This “normotensive CS” is commonly seen in patients with bradycardia, and can lead to false reassurance.
- When managing CS, clinicians need to look for markers of perfusion in addition to patient’s BP because BP can be misleading.
Assessment of a patient with suspected CS
- Approach to patient with suspected CS:
- Assess for end-organ perfusion / vasoconstriction
- Assess for R-sided and L-sided congestion
- Identify causes of CS
- Identify end-organ malperfusion and vasoconstriction
- Physical exam:
- Tachycardia, hypotension, reduced pulse pressure
- Cold/clammy extremities, reduced capillary refill, altered mental state
- **Reduced urine output** (directly related to renal perfusion and cardiac output)
- < 30ml/min is concerning, but usually CS patients are anuric.
- Laboratory Markers of Malperfusion:
- Elevated Lactate
- Elevated Liver Enzymes (liver congestion/malperfusion)
- Elevated Troponin (reduced coronary perfusion)
- Elevated CK (poor muscle perfusion)
- **Reduced Central Venous Oxygenation (CvO2) < 70%**
(increased O2 extraction by tissues)- CvO2 is obtained from a blood gas drawn from a central line, and is very helpful in diagnosis of CS. Patients with sepsis have reduced tissue O2 extraction and should have an elevated or normal CvO2.
- Elevated Creatinine (late finding – may take days to rise)
- Invasive hemodynamics
- Reduced Cardiac Output (CO) < ~4 L/min (Cardiac Index (CI) < 2.2 L/min/m^2)
- High systemic vascular resistance (SVR)
- NOTE: Low SVR is not compatible with CS, and suggests vasodilatory shock (i.e. sepsis)
- Physical exam:
- Identify markers of congestion
- Physical Exam:
- R-sided –> Peripheral edema, Ascites, Pulsatile Liver
- L-sided –> Pulmonary Rales / Effusions
- Laboratory:
- BNP is helpful for risk-stratification, but is non-specific in CS. Although, low BNP suggests an alternative diagnosis.
- Chest Xray: Pulmonary edema/Effusions
- Invasive Hemodynamics
- Elevated right atrial pressure (RAP)
- Elevated left atrial pressure – (surrogate is Pulmonary Capillary Wedge Pressure PCWP)
- Elevated left ventricular end-diastolic pressure (LVEDP) > 15 mmHg
- Practical Tip: **LVEDP is often available in the cardiac angiogram report** if the interventional cardiologist enters the LV cavity.
- Physical Exam:
- Identify Causes of CS
- Physical Exam:
- New murmur? (Acute MR/AR)
- Arrhythmias
- Signs of tamponade (muffled sounds, pulsus paradoxus)
- Laboratory: Troponin is non-specific.
- ECG: Acute STEMI? Arrhythmia? (everything else is non-specific)
- Echocardiogram:
- An urgent echocardiogram is indicated since it identifies most causes and can reveal urgent treatment options.
- Physical Exam:
- If the patient is congested, preload must be cautiously lowered to reduce congestion and improve oxygenation, while minimizing the reduction in stroke volume.
Classes of CS
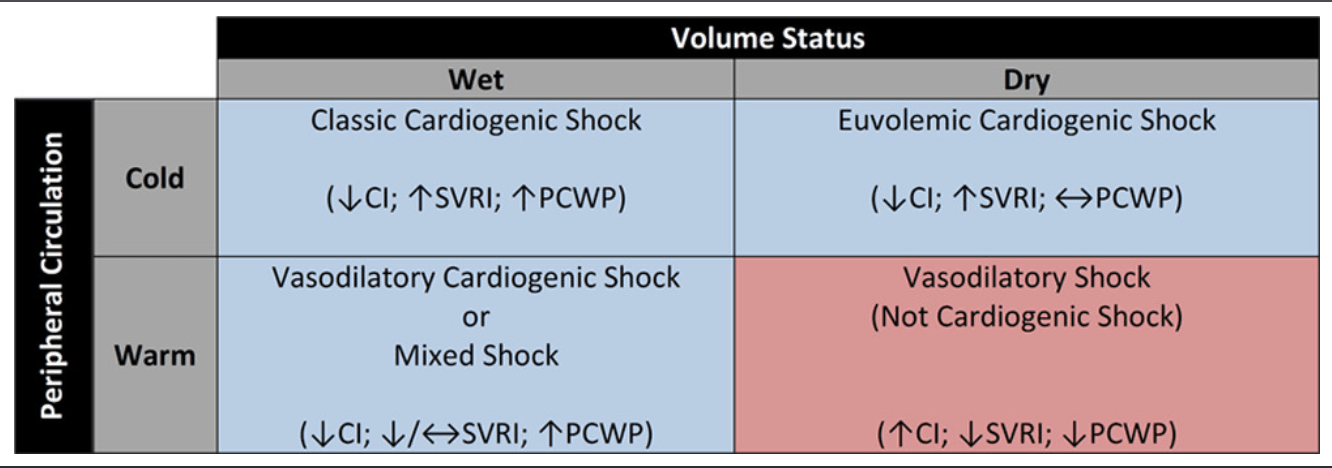
- Your assessment should place a patient with CS into one of the 4 categories (see diagram on the right).
- NOTE: Warm/Cold is related to vasoconstriction/dilation, and wet/dry refers to venous/filling pressures (preload).
- Cold + Wet –> Most CS patients with congestion and poor perfusion
- Management includes decongestion.
- Cold + Dry –> Reduced cardiac output + normal/reduced filling pressures (preload).
- Seen in patients with CS who are decongested (i.e. diuresis)
- Further decongestion may worsen hemodynamics. Management must focus on increasing CO.
- Warm + Wet –> Cardiogenic shock with vasodilation / mixed shock (commonly seen in CS patients with overlying sepsis).
- Warm + Dry –> Classic vasodilatory shock (not CS).
- Cold + Wet –> Most CS patients with congestion and poor perfusion
Invasive Hemodynamics
- Swan-Ganz catheter used to objectively measure hemodynamic parameters, which can be used to:
- Confirm diagnosis of CS.
- Determine hemodynamic response and guide titration of supportive medical therapy.
- Risk stratify CS patients and determine eligibility and type of advanced heart failure therapy (i.e. cardiac transplantation, mechanical ventricular support, and palliative care).
- Practical Tip: #3 is the most important reason for Swan-Ganz Catheterization. A patient in CS may be temporarily supported by altering hemodynamics with diuretics, vasopressors, and inotropes. However, identifying and delivering definitive advanced therapy is the only long-term solution.
- In patients with cardiogenic shock the use of balloon flotation catheters to determine the hemodynamic response to supportive therapy is still necessary and strongly recommended.
Scan-Ganz Catheter
Approach
- Volume Management
- Often very difficult to assess volume status, esp. in AMI
- Some patients may benefit from intravenous volumes esp. RV infarct patients since they are preload dependent. Others benefit from diuresis “cold and wet” or frank pulmonary edema.
- Definitive invasive measurement of filling pressures with RHC recommended for patients with unclear cause of shock or volume status
- Hemodynamic monitoring
- Art line, Cardiac monitor, SpO2, Temperature, Urine output, Mixed venous blood gas (+/- pulmonary artery catheter)
- Oxygenation & ventilation
- If intubation required, low tidal volume (5-7ml/kg) –> decreased incidence of right ventricular failure.
- Positive pressure ventilation may worsen RV failure since it will increase pulmonary vascular resistance (PVR) and worsen pulmonary pressures -> in this case target low PEEP strategy
- Positive Pressure ventilation may help isolated LV dysfunction since will reduce afterload and decrease preload which would be beneficial in patient with high systemic vascular resistance (SVR) and volume overloaded patients
- Hemodynamic goals
- Vary from patient to patient
- No umbrella suggestion
- Consider risks of increased support vs. increased cardiac demand and arrhythmia risk
- Patient-specific titration of support to adequacy of end-organ perfusion
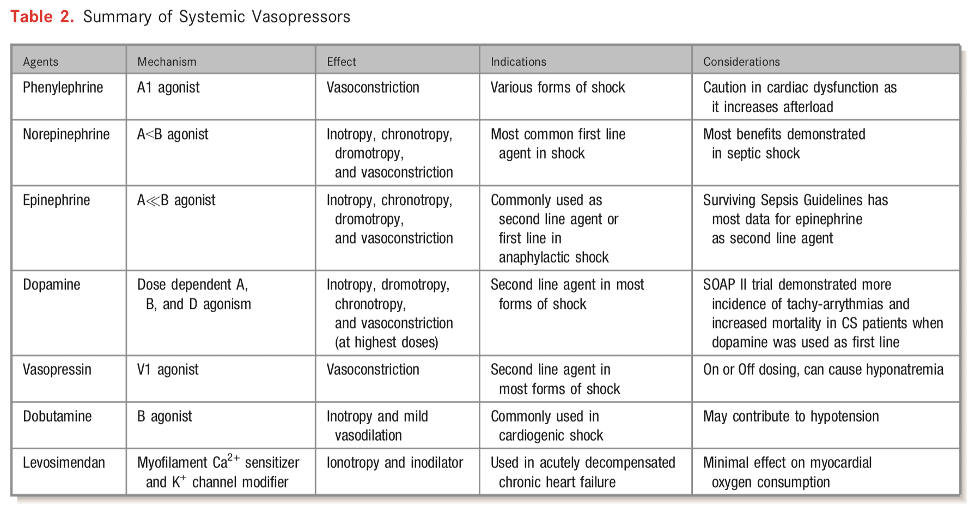
- Vasopressor support
- Very few clinical trials evaluating vasopressors/inotropes in CS
- Norepinephrine generally first line agent
- SOAP II trial — norepinephrine as first line agent in patients with shock was associated with less arrhythmia vs. dopamine [in the CS subgroup]
- There has been a small randomized trial comparing norepinephrine vs epinephrine in cardiogenic shock from MI (Pump Dysfunction) and found that norepinephrine was superior to epinephrine. Epinephrine resulted in more refractory shock cases and worsening metabolics including lactic acidosis but no mortality difference between the groups.
Add Your Heading Text Here
Milrinone vs Dobutamine
Typically, patients are started an ionodilator such as milrinone and dobutamine. There are important considerations when using these medications that may sway a clinician to use one or the other.
- Onset/Half-life:
- Dobutamine has a very quick onset (1-10minutes) and a very short half-life (2 minutes). This has pharmacological advantages for being able to titrate therapy and discontinue therapy should the patient’s hemodynamics dramatically change (i.e. Hypotension).
- Milrinone on the other hand slightly longer onset of action (5-15 minutes) but more importantly has a very long half-life (2.4 hours) this is even longer in patients with renal dysfunction.
- Hypotension/Reduction in SVR:
- Generally, Milrinone causes more hypotension and is more potent at reducing systemic vascular resistance than Dobutamine.
- This may be a desired effect in a cardiogenic shock patient if hypertensive or with profoundly elevated SVR.
- Renal Failure:
- Milrinone is renally cleared and needs to be renally adjusted.
- There is a risk of Milrinone accumulation which can lead to severe hypotension.
- Beta- Blocker Use:
- Milrinone is generally preferred in patients on chronic beta-blockers since their use causes down-regulation of beta-receptors and will make Dobutamine less effective.
- This can sometimes be overcome by a higher dose of Dobutamine.
Hemodynamic Approach
- There is no one way or “cookie-cutter” approach to managing cardiogenic shock.
- Important considerations include Isolated right ventricular dysfunction, Isolated left ventricular dysfunction, biventricular failure, pulmonary hypertension, etiology of shock, arrythmias, valvular disease, these factors drastically effect how cardiogenic shock is managed including therapeutic options that can be provided to patients.
- See table to right/below from AHA that describes tailoring vasoactive management depending on phenotype
- Preferred Pressor: There may be times that a specific pressor is desired. This is generally a non-evidence based approach but often relies on pharmacological principles. For example, a patient with severe or acute pulmonary hypertension with hypotension, Vasopressin may be the preferred agent of choice since it will increase blood pressure but not effect pulmonary vascular resistance.
Renal Failure
Further Reading
- Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Sean Van Diepen et. al. 2017. (html)
- Cardiogenic Shock. American Heart Association. Valdahpour et al. 2019. (html)
- SCAI Clinical Expert Consensus Statement on the Classification of Cardiogenic Shock. Catheterization and Cardiovascular Interventions. David Baren et al. 2019. (html)
- Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction. Journal of American College of Cardiology. Bruno Levy et al. 2018. (html)
Authors
- Authors: Dr. Daniel Durocher (MD, FRCPC, ICU Fellow)
- Reviewer: Atul Jaidka (MD, FRCPC, Cardiology Fellow)
- Staff Reviewer: Pending (MD, FRCPC[Cardiology])
- Last Updated: May 7, 2021
- Comments or questions please email feedback@cardioguide.ca