Abbreviations
- PPM – Pacemaker
- CRT – Cardiac Resynchronization Therapy
- ICD – Implantable Cardioverter Defibrillator
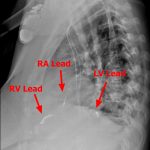
- RV – Right Ventricle
- LV – Left Ventricle
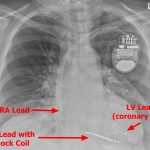
- RA – Right Atrium
Basics of Devices
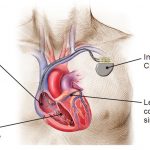
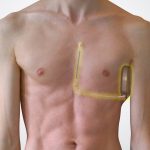
- Most devices have 2 components: Pulse Generator and Leads
- The leads are composed of silicone or polyurethane polymer, and have a retractable helix on the end to fix into the myocardium.
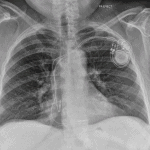
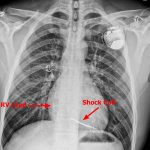
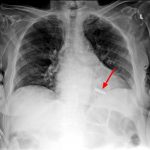
- Clinicians who read chest X-rays should be able to recognize types of devices on chest radiographs
Pacemaker
- Implanted device for the treatment of bradycardia
TYPES: - Single chamber – Single lead in the RV
- Dual chamber – Lead in the RA appendage as well as RV. This allows RV pacing in response to sensed atrial activation (tracking) as well as atrial pacing if the patient has sinus node dysfunction. Dual chamber pacemakers maintain atrio-ventricular synchrony, which may be important in some patients.
Implantable Cardioverter Defibrillator (ICD)
- ICDs have a high-voltage lead with a shock coil (radio-opaque distal end), which allows treatment of ventricular arrhythmias (called “tachycardia therapy”).
- Tachycardia therapy consists of 2 interventions:
- Anti-tachycardia pacing (ATP), which allows the device to pace rapidly to terminate arrhythmia
- Cardioversion/defibrillation if the tachycardia is dangerously fast or ATP is unsuccessful.
- All ICD devices are also able to pace in the event of bradycardia, but pacing is not preferred and is usually set very low (i.e. backup at 30-40bpm) because RV pacing can be harmful in patients with heart failure and reduced EF.
TYPES: - Single Chamber ICD – Single high-voltage RV lead
- Dual Chamber ICD – High voltage RV lead and a regular pacing RA lead. This allows additional atrial pacing in patients with bradycardia
Cardiac Resynchronization Therapy (CRT)
- Patients with reduced EF and a wide QRS (either from RV pacing or LBBB) can have very poor heart failure outcomes. This is thought to be caused by dyssynchrony in the left ventricular activation, which worsens mitral regurgitation due to dyssynchrony of papillary muscles and left ventricular remodeling characterized by progressive dilation and LV systolic dysfunction.
- CRT is intended to pace the heart from two locations – the RV apex and the postero-lateral wall of the LV simultaneously. This resynchronizes activation of the LV and improves heart failure outcomes.
- NOTE: The LV is accessed through the coronary venous system – by placing the lead into the posterolateral coronary vein via the coronary sinus.
TYPES: - CRT-D – Has 3 leads – an atrial pacing lead, RV defibrillation/pacing lead, and LV pacing lead.
- CRT-P – Same as CRT-D, except all 3 leads are pacing leads with no ability to shock.
Special Devices
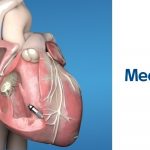
- Leadless pacemaker (i.e. Medtronic Micra)
- Inserted through the femoral vein into the RV. The entire pacemaker is ~3-4cm in size, and operates in the RV.
- Advantages:
- Very high resistance to infection due to absence of leads, which reduces vascular surface area (One documented infection in 50,000 implants in post-marketing studies).
- No leads – less risk of lead malfunction (fracture, etc.)
- Minimally invasive, rapid recovery – no incision or pocket
- Disadvantages:
- Slightly shorter battery life (~8 years)
- Technically challenging, only done in specialized centres
- Single chamber only (cannot track atrial contraction or pace atria in sinus node dysfunction). Although new “AV Micra” is able to detect mechanical atrial contract.
- Subcutaneous ICD
- ICD generator implanted on the lateral chest wall, and a lead is tunneled subcutaneously to the xyphoid process and then anterior to the sterum.
- Advantages: No vascular leads (lower infection risk)
- Disadvantages: Large device, cannot pace (no ability for anti-tachycardia pacing (ATP))
Managing a patient awaiting device implantation
- NPO at midnight
- Device implants are performed in semi-awake state with procedural sedation.
- Hold anticoagulation (but continue warfarin uninterrupted)
- BRUISE-CONTROL study demonstrated the safety of performing device implantation with a therapeutic INR. Patients who had warfarin interruption and bridged with heparin had a higher rate of significant bleeding compared to warfarin continuation. However many operators will not perform device procedures with INR > 3.0 due to risk of hematoma.
- BRUISE-CONTROL II study demonstrated safety in continuing DOACs. However, some operators prefer to hold 2-3 doses to minimize risk of bleeding and accept negibible clinical impact of interrupting a few doses of DOAC. This is centre dependent and if unsure confirm local practices.
- Stabilize the patient
- Patients with recurrent asystole or hemodynamic instability are poor candidates for procedures. They should be stabilized with temporary transvenous pacing and require treatment of other acute illnesses prior to permanent device implantation.
- It is important to remember that device implantation is rarely an emergency, and can be done at any time throughout patient’s hospital stay.
- Pre-Device Workup
- Generally patients require an echocardiogram prior to device implantation because the type of device may be affected by the patient’s LV systolic function (i.e. if a patient with AV block needs ICD or CRT)
Post device implantation
- All patients get a post-procedure chest X-ray to detect pneumothorax, lead dislodgement, and an ECG
- Pacemakers receive an additional ECG with magnet applied over the generator
- Avoid getting the incision wet for 48 hours. Patients can shower, but must keep the incision dry.
- For suprapectoral implants: Avoid lifting the arm above the level of the shoulder for 4 weeks (avoids generator dislodgement).
- Apply driving guidelines (no driving 1 week post-pacemaker, and 1 month post-ICD, unless longer restriction is indicated)
- ICD Advice: If a patient receives a single shock and feels otherwise well, they should contact the ICD clinic when they are open for an appointment (no need to go to ER for shock alone). If the patient receives 2 or more shocks, they should go straight to ER for urgent evaluation.
Cardiac Device Troubleshooting
Magnet Mode
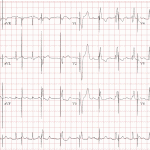
- Magnet mode is critical to managing patients with suspected device malfunction and those with VT storm.
- If there is a concern about pacemaker malfunction, a clinician can obtain ECG with a magnet applied to the pacemaker. This will show asynchronous pacing (blind pacing no matter what) as long as the magnet is applied. Magnet should be removed after assessment to avoid competition with intrinsic rhythm (see figure)
- Patients with an ICD and VT storm can have a magnet applied to disable shocking and ATP therapy. This will allow the clinician to examine the patient’s rhythm, deliver anti-arrhythmic drugs and shock externally if required. Removing the magnet will re-enable ventricular arrhythmia detection and therapy.
- All cardiac implanted devices have a reed switch, which allows “magnet mode operation” when a magnet is applied. All ERs and code blue resuscitation carts have a circular magnet for this purpose.
| Device | Magnet Mode Operation |
|---|---|
| Pacemakers | Asynchronous (VOO or DOO) pacing at 80bpm (device is blinded, and will pace no matter what) |
| ICD | No effect on pacing function. Disables tachycardia detections/treatments, and will not perform ATP/shock while magnet is applied. |
| CRT-P | Same as pacemakers |
| CRT-D | Same as ICD |
- NOTE: Different device manufacturers can have different magnet mode operations. The above table represents most common responses between manufacturers. It is best to review specific manufacturer manuals to determine magnet mode operation.
Contributors
- Author: Dr. Pavel Antiperovitch (MD, FRCPC Cardiologist, EP Fellow)
- Reviewer: Dr. Atul Jaidka (MD, FRCPC Internal Medicine, Cardiology Fellow)